What is Metal?
"Metal" is an almost impossibly broad term that takes in everything from lead (a super-heavy metal) and aluminum (a super-light one) to mercury (a metal that's normally a liquid) and sodium (a metal soft enough to cut like cheese that, fused with chlorine, you can sprinkle on your food—as salt!).
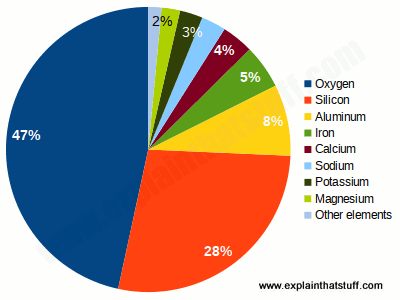
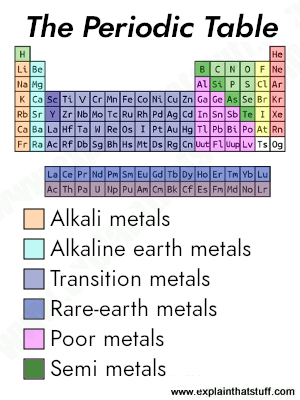
You might think Earth is a big lump of rock, hard on the outside and soft in the middle—but quite a lot of it is actually metal. What exactly is metal? Over three quarters of the chemical elements that occur naturally on our planet are metals, so it's almost easier to say what metal isn't.
When we talk about metals, we're usually referring to chemical elements that are solid (with relatively high melting points), hard, strong, durable, shiny, silvery gray in color, good conductors of electricity and heat, and easy to work into various different shapes and forms (such as thin sheets and wires). The word metal is quite a broad and vague term, and not something you can define precisely.
Alloys
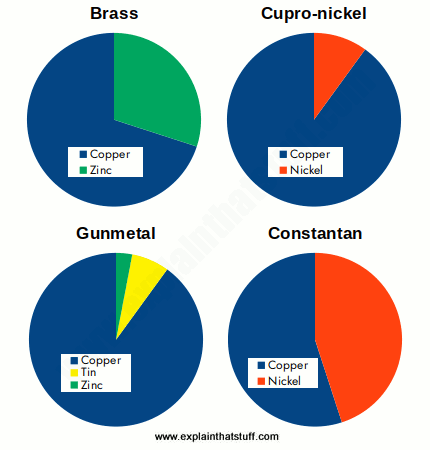
When is a metal not a metal? Look at a car, plane, or motorcycle and you see lots of metal—or do you? Quite a lot of the metal-like materials around us are actually alloys: metals that have been mixed with other materials (metals or nonmetals) to make them stronger, harder, lighter, or superior in some other way. Steel is an alloy of iron, for example, that contains a small amount of carbon (different types of steel contain more or less carbon). Bronze is an alloy of copper and tin, while brass is the alloy you get when you mix copper and zinc.
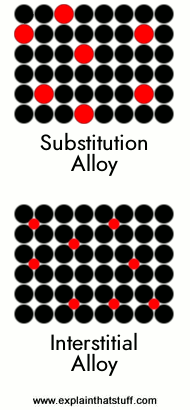
If you look at a metal through a powerful electron microscope, you can see the atoms inside arranged in a regular structure called a crystalline lattice. Imagine a small cardboard box full of marbles and that's pretty much what you'd see. In an alloy, apart from the atoms of the main metal, there are also atoms of the alloying agents dotted throughout the structure. (Imagine dropping a few plastic balls into the cardboard box so they arrange themselves randomly among the marbles.)
|
If the atoms of the alloying agent replace atoms of the main metal, we get what's called a substitution alloy. An alloy like this will form only if the atoms of the base metal and those of the alloying agent are of roughly similar size. In most substitution alloys, the constituent elements are quite near one another in the periodic table. Brass, for example, is a substitution alloy based on copper in which atoms of zinc replace 10–35 percent of the atoms that would normally be in copper. Brass works as an alloy because copper and zinc are close to one another in the periodic table and have atoms of roughly similar size.
Alloys can also form if the alloying agent or agents have atoms that are very much smaller than those of the main metal. In that case, the agent atoms slip in between the main metal atoms (in the gaps or "interstices"), giving what's called an interstitial alloy. Steel is an example of an interstitial alloy in which a relatively small number of carbon atoms slip in the gaps between the huge atoms in a crystalline lattice of iron. |
People make and use alloys because metals don't have exactly the right properties for a particular job. Iron is a great building material but steel (an alloy made by adding small amounts of nonmetallic carbon to iron) is stronger, harder, and rustproof. Aluminum is a very light metal but it's also very soft in its pure form. Add small amounts of the metals magnesium, manganese, and copper and you make a superb aluminum alloy called duralumin, which is strong enough to make airplanes. Alloys always show improvements over the main metal in one or more of their important physical properties (things like strength, durability, ability to conduct electricity, ability to withstand heat, and so on). Generally, alloys are stronger and harder than their main metals, less malleable (harder to work) and less ductile (harder to pull into wires).
Examples of Copper Alloys
FERROUS VS NON FERROUS METALS (WHAT IS THE DIFFERENCE?)
The difference between ferrous and non-ferrous metals is that ferrous metals contain iron and non-ferrous metals do not.
|
Ferrous metals are any metal that contains iron, such as stainless steel. They are known for their tensile strength, which makes them ideal for architectural and structural uses such as the tallest skyscrapers, as well as bridges, railways and more.
Ferrous metals are also have magnetic properties, which is why you can use magnets to pin things to your refrigerator door, although their high carbon content means that many ferrous metals are prone to rusting. The exceptions to this are stainless steel, which doesn’t rust because of the chromium, and wrought iron which doesn’t rust due to the high pure iron content. Examples and Uses of Ferrous Metals Commonly used examples of ferrous metals include steel, stainless steel, carbon steel, cast iron and wrought iron: 1. Steel A combination of iron and carbon, steel is renowned for its strength and machinability. It is widely used in construction, manufacturing and industrial metal fabrication. 2. Stainless Steel Stainless steel is an alloy steel made with the addition of chromium to steel, which provides resistance against rust. 3. Carbon Steel Carbon steel contains a high carbon content that is added to iron to create an exceptionally hard metal that is used for tools. 4. Cast Iron Cast iron is a hard and wear resistant metal that is widely used for items including cookware, machine tools, engines, manhole covers and water pipes. 5. Wrought Iron Unlike most other ferrous metals, wrought iron is able to resist corrosion and oxidation. It is typically used for fences, railings and gates. |
Non-ferrous metals don’t contain iron. They are lighter and more malleable than ferrous metals, making them ideal for applications where strength is required but weight is a consideration, such as with the aerospace industry.
Non-ferrous metals are not magnetic but do offer good resistance to corrosion and can conduct heat and electricity. They are used in for items including industrial piping, gutters, roofing and electrical applications. Examples and Uses of Non Ferrous Metals Commonly-used non-ferrous metals include aluminum, lead, silver, brass, gold, zinc, copper and tin: 1. Aluminium Lightweight and easy to machine, shape and weld, aluminum is used for a range of applications from food cans and cookware to airplane parts and cars. 2. Copper A good conductor of heat and electricity, copper is highly ductile and malleable. It is widely used for electrical wiring as well as in appliances and vehicles. 3. Lead With a low melting point and low tensile strength, lead is used in electrical power cables, batteries, pipes, fuels, paint and for soldering. 4. Tin Soft and malleable with a low tensile strength, tin is used as a coating to prevent steel from corroding. 5. Silver Silver is used for a range of applications, including jewelry, cutlery, electrical contacts and in mirrors. 6. Brass Brass is used for fixtures and fittings including taps, hooks, and doorknobs, as well as being used for light fittings and screws, among other uses. 7. Gold Used for jewelry, gold also has applications including within the medical industry, in computers and also electronics. 8. Zinc A medium strength metal with a low melting point, zinc is used to galvanise iron and steel to prevent rusting. |
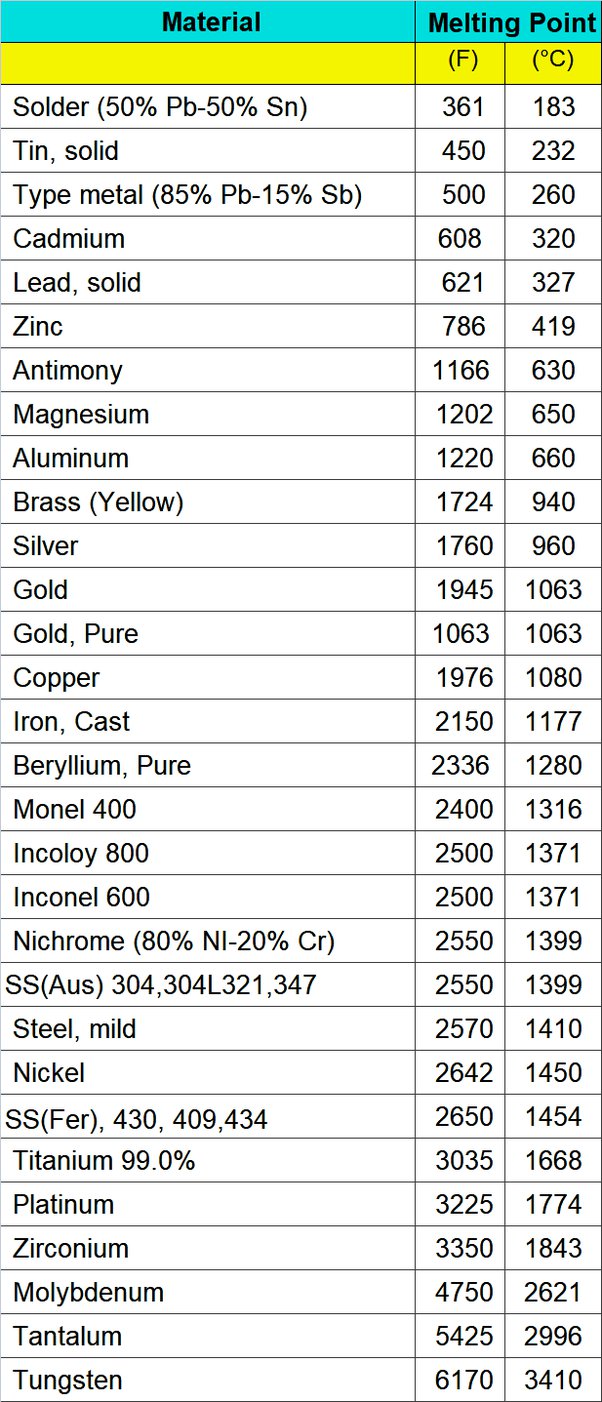
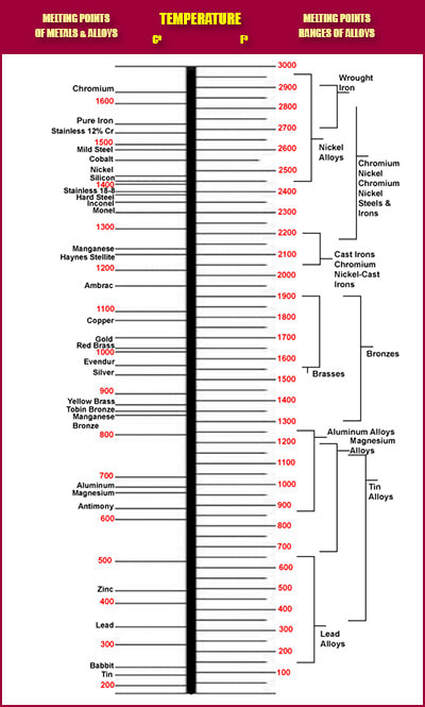
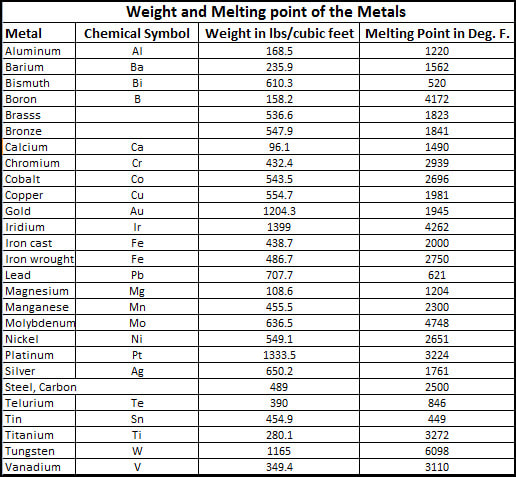
Melting Points of Metals
WHAT IS ANNEALED METAL?
Annealing is a specific process of heat treatment that alters the properties of metal. While there are many different types of heat treatment, annealing is popular because it increases ductability and reduces hardness.
What Is The Annealing Process?
Annealing is a heat treatment process that is common in manufacturing because it improves the physical and sometimes chemical properties of metal to be both more durable and more workable. When heated during the specific process of annealing, atoms migrate in their crystal lattice and the number of atom dislocations goes down, leading to changes in both ductility and hardness. As the material cools it crystallizes again.
For many alloys, including the most common in manufacturing, carbon steel, the properties of the metal are determined by the size of the crystal grains and the phase composition. Both change during heating and cooling. With knowledge of the crystal grain composition and the phase diagram, annealing as a heat treatment can be used to take the metal from hard to soft, brittle to ductile. As a result, the metal will be more formable, an obviously favorable property in manufacturing.
What is the Benefit to Annealing?
As already shared, annealing is used to make metal more ductile and less brittle.
Here are the three main benefits to annealing:
What Metals Are Commonly Annealed?
Most commonly, many types of steel and cast iron are annealed in the manufacturing industry. There are also specific types of aluminum, copper, and brass that can be annealed. While steel is generally cooled to room temperature in still air, copper and brass can also be quenched in water.
What Is The Annealing Process?
Annealing is a heat treatment process that is common in manufacturing because it improves the physical and sometimes chemical properties of metal to be both more durable and more workable. When heated during the specific process of annealing, atoms migrate in their crystal lattice and the number of atom dislocations goes down, leading to changes in both ductility and hardness. As the material cools it crystallizes again.
For many alloys, including the most common in manufacturing, carbon steel, the properties of the metal are determined by the size of the crystal grains and the phase composition. Both change during heating and cooling. With knowledge of the crystal grain composition and the phase diagram, annealing as a heat treatment can be used to take the metal from hard to soft, brittle to ductile. As a result, the metal will be more formable, an obviously favorable property in manufacturing.
What is the Benefit to Annealing?
As already shared, annealing is used to make metal more ductile and less brittle.
Here are the three main benefits to annealing:
- Annealing makes metals more formable. When metal is stronger and more ductile, it gives manufacturers more leeway in the fabrication process. There is less risk of material fracturing from bending or pressing.
- Annealing can also improve a metal’s ability to be machined and improve the lifespans of tools. Hard, brittle metals can cause wear to shop tools. Annealing metals reduces wear and the chance of damage to tools.
- Annealing removes what’s called residual stress. Residual stress is what remains in a metal after the original cause of the stress has been removed. For example, residual stress from roll forming could cause a structural to gape when cut with a band-saw. Residual stress can complicate future processes and annealing is a great way to remove it.
What Metals Are Commonly Annealed?
Most commonly, many types of steel and cast iron are annealed in the manufacturing industry. There are also specific types of aluminum, copper, and brass that can be annealed. While steel is generally cooled to room temperature in still air, copper and brass can also be quenched in water.
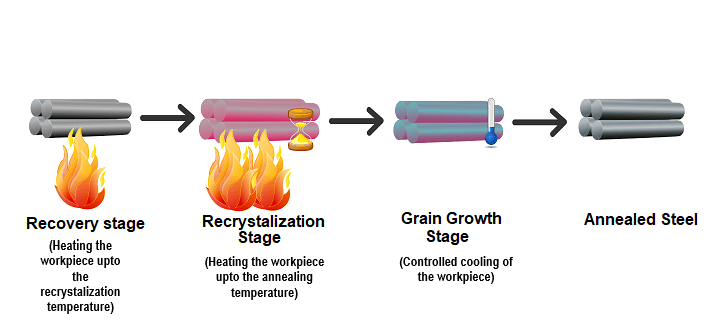
What Are The Steps in The Annealing ProcessThere are three main steps in the annealing process:
- Recovery
- Recrystallization
- Grain growth
Recovery
Metal is composed of a lattice of crystal structures that are known as grains. Sometimes, the structure of the grains themselves cause stress to the metal. During the first phase of the annealing process, called recovery, a furnace or other type of heat source is utilized to raise the temperature of the material to a point that removes internal stresses.
Recrystallization
During recrystallization, further heating raises the temperature of the metal to just below its melting point, high enough that the atoms recrystallize, and low enough that it doesn’t melt.
Grain Growth
During the grain growth stage, new crystal grains become fully developed as the metal cools that don’t have the metal’s original stress. The final composition–including the ductility and hardness–is determined by the rate of cooling. Once the metal is annealed, there may be final processing like shaping, stamping, or forming.
Metal is composed of a lattice of crystal structures that are known as grains. Sometimes, the structure of the grains themselves cause stress to the metal. During the first phase of the annealing process, called recovery, a furnace or other type of heat source is utilized to raise the temperature of the material to a point that removes internal stresses.
Recrystallization
During recrystallization, further heating raises the temperature of the metal to just below its melting point, high enough that the atoms recrystallize, and low enough that it doesn’t melt.
Grain Growth
During the grain growth stage, new crystal grains become fully developed as the metal cools that don’t have the metal’s original stress. The final composition–including the ductility and hardness–is determined by the rate of cooling. Once the metal is annealed, there may be final processing like shaping, stamping, or forming.